potatis is salt vatten
hej
Jag vett när vi sättas potatis i slat vatten sker osmos !
Men kommer sker lite diffusion och endocytosis and exocytosis för att cellen försokar att hålla homeostasis ( där är förhällanden i cell stabilt för cells funktioner)????eller det är fel.
Det som sker är diffusion av vatten, som rör sig mot den volymen med högst koncentration av osmotiskt aktiva ämnen. I ditt fall sker vattnets flöde från potatisens celler (lägre koncentration) till det salta vattnet (med högre koncentration).
Endo-/exocytos är för långsamma processer för att riktigt påverka osmosen.
tack för din svar.
What I am trying to say that. if we compare the concentration of salt in the water and in the cell , The concentration of salt will be higher in the water than in the cell. so ions channel will may transport some ions to the cell but then close because the cell does not want more ions into the cell..
Am I right?
yes I agree what u said above that osmos is happening ( as I said before) But there are maybe a very small ions also has been transported.
The definition of osmosis is (pretty much) the net movement/diffusion of solvent molecules across a semi-permeable membrane, as a spontaneous action.
Hence, the any would be ions are not part of the osmosis.
Thank you again.
I understand the difference between osmosis and diffusion.
maybe it is my fault for explain my question very clearly.
Here i go.
let say we put cell inside salt soultion (NaCl) which has higher concentration of ion (Na+) and (Cl-).
the step that i belive could happen are.
1. At first few seconds, the cell has all there ions channel open ( if not all almost) especially aquaporins
2. water will start to go out from the cell
3. some ion may enter the cell which will increases the concentration of ion (Na+) and (Cl-). that will force the cell to close its ion channels because the transport process has one goal is to keep the condiction inside the cell very good for cell function.
4. the cell will stop sending water out of the cell by closing the aquaporins but it the cell was not able to stop the water from slipping away then the cell will be damaged the cell.
Would you please critic me if i am wrong.
Note: this is not homework. it is my thought I am trying to figure out what exactly happed. Where other student will only will discuss osmosis without talking in consideration that cell membran has aslo ion channels beside the aquaporins .
Note: as far as I understand that Endo-/exocytos only work with big molecules and particles. Is there any chance that they can occur with ions.
Stephan70707 skrev:Thank you again.
I understand the difference between osmosis and diffusion.
maybe it is my fault for explain my question very clearly.
Here i go.
let say we put cell inside salt soultion (NaCl) which has higher concentration of ion (Na+) and (Cl-).
the step that i belive could happen are.
1. At first few seconds, the cell has all there ions channel open ( if not all almost) especially aquaporins
No, ion channels do not open in response to a higher concentration of ions outside the cell. You are probably mixing this up with the ion conductance channels we have in the nerve cells of our bodies. One type of these the voltage gated channels do respond to a change in voltage over the membrane, by opening. This opening is however resulting from an electric effect (as during effect e.g. a nerve impulse). A concentration difference of does not result in a electric effect, in particular as the respective charges on the outside are balanced.
Aquaporins on the other are always open, and do thereby allow water molecules to diffuse out of the cells into the surrounding solution with a higher concentration of salt - i.e. osmosis occurs.
2. water will start to go out from the cell
Yes.
3. some ion may enter the cell which will increases the concentration of ion (Na+) and (Cl-). that will force the cell to close its ion channels because the transport process has one goal is to keep the condiction inside the cell very good for cell function.
No, the cellular membrane prevents the diffusion of ions. And as there is no activation/opening of Na/Cl channels - there will be no flow of ions into the potato cells.
4. the cell will stop sending water out of the cell by closing the aquaporins but it the cell was not able to stop the water from slipping away then the cell will be damaged the cell.
The aquaporins cannot be closed, so water will keep escaping the cell by osmosis, till the water concentration has been equilibrated (or more precise till osmosis stops, as a consequence the amount of osmotically active particles is equal on the respective sides of the cellular membrane).
Would you please critic me if i am wrong.
Note: this is not homework. it is my thought I am trying to figure out what exactly happed. Where other student will only will discuss osmosis without talking in consideration that cell membran has aslo ion channels beside the aquaporins .
Note: as far as I understand that Endo-/exocytos only work with big molecules and particles. Is there any chance that they can occur with ions.
Sure, some ions are caught during e.g. endocytosis, as these ions are present in the liquid outside the cell and will inevitably end up inside the formed vesicle, but this is on such a minute level, that it does not matter.
I am so grateful for u :)
thanks for your answer but here my arguments:
please understand that i am trying to figure out and use university page to find those answer because i want to get an A so i know i am maybe taking things too far.
But I am studying by myself :)
mag1 skrev:Stephan70707 skrev:Thank you again.
I understand the difference between osmosis and diffusion.
maybe it is my fault for explain my question very clearly.
Here i go.
let say we put cell inside salt soultion (NaCl) which has higher concentration of ion (Na+) and (Cl-).
the step that i belive could happen are.
1. At first few seconds, the cell has all there ions channel open ( if not all almost) especially aquaporins
No, ion channels do not open in response to a higher concentration of ions outside the cell. You are probably mixing this up with the ion conductance channels we have in the nerve cells of our bodies. One type of these the voltage gated channels do respond to a change in voltage over the membrane, by opening. This opening is however resulting from an electric effect (as during effect e.g. a nerve impulse). A concentration difference of does not result in a electric effect, in particular as the respective charges on the outside are balanced.
Aquaporins on the other are always open, and do thereby allow water molecules to diffuse out of the cells into the surrounding solution with a higher concentration of salt - i.e. osmosis occurs.
In book IRIS biologi 2 (sidan 70) says:" Olika kanalproteiner är anpassade för transport av speciella ämnen och vanligen kan de öppnas eller stängas efter behov. Kanaler för joner kallas även jonkanaler."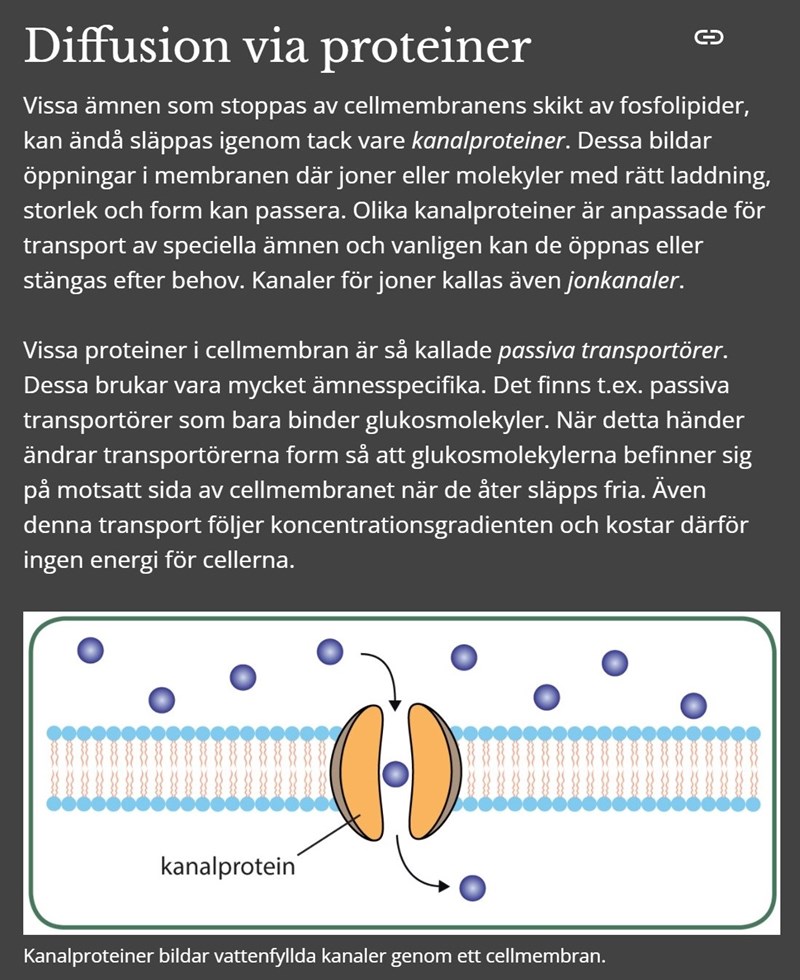
2. water will start to go out from the cell
Yes.
3. some ion may enter the cell which will increases the concentration of ion (Na+) and (Cl-). that will force the cell to close its ion channels because the transport process has one goal is to keep the condiction inside the cell very good for cell function.
No, the cellular membrane prevents the diffusion of ions. And as there is no activation/opening of Na/Cl channels - there will be no flow of ions into the potato cells.
this point i am very confused with. where the cell have ion channels which is open in normal conditions. the cell is placed in the salt solution may get in ( due to diffusion) but then close immediately because the cell will want to have more than what it need of ions so no harm came from having to much ions
4. the cell will stop sending water out of the cell by closing the aquaporins but it the cell was not able to stop the water from slipping away then the cell will be damaged the cell.
The aquaporins cannot be closed, so water will keep escaping the cell by osmosis, till the water concentration has been equilibrated (or more precise till osmosis stops, as a consequence the amount of osmotically active particles is equal on the respective sides of the cellular membrane).
according to Illinois university (https://www.ks.uiuc.edu/Research/aquaporins/) Gating of Water Channels so what do u think.
Would you please critic me if i am wrong.
Note: this is not homework. it is my thought I am trying to figure out what exactly happed. Where other student will only will discuss osmosis without talking in consideration that cell membran has aslo ion channels beside the aquaporins .
Note: as far as I understand that Endo-/exocytos only work with big molecules and particles. Is there any chance that they can occur with ions.
Sure, some ions are caught during e.g. endocytosis, as these ions are present in the liquid outside the cell and will inevitably end up inside the formed vesicle, but this is on such a minute level, that it does not matter.
Stephan70707 skrev:
mag1 skrev:Stephan70707 skrev:Thank you again.
I understand the difference between osmosis and diffusion.
maybe it is my fault for explain my question very clearly.
Here i go.
let say we put cell inside salt soultion (NaCl) which has higher concentration of ion (Na+) and (Cl-).
the step that i belive could happen are.
1. At first few seconds, the cell has all there ions channel open ( if not all almost) especially aquaporins
No, ion channels do not open in response to a higher concentration of ions outside the cell. You are probably mixing this up with the ion conductance channels we have in the nerve cells of our bodies. One type of these the voltage gated channels do respond to a change in voltage over the membrane, by opening. This opening is however resulting from an electric effect (as during effect e.g. a nerve impulse). A concentration difference of does not result in a electric effect, in particular as the respective charges on the outside are balanced.
Aquaporins on the other are always open, and do thereby allow water molecules to diffuse out of the cells into the surrounding solution with a higher concentration of salt - i.e. osmosis occurs.
In book IRIS biologi 2 (sidan 70) says:" Olika kanalproteiner är anpassade för transport av speciella ämnen och vanligen kan de öppnas eller stängas efter behov. Kanaler för joner kallas även jonkanaler.
Yes these channels are essential for establishing and maintaning the normal state of the cell (the so called cellular homoeostasis). These channels do indeed transport ions through the membrane, but in a strictly regulated way by tight regulation - as not to disrupt the homeostasis. But these channels, and the transport systems they are part of, are not capable of coping with this type of (salt induced) osmosis.
These channels are either opening/closing to sustain the homeostasis (e.g. leak channels in neurons, trying to keep a stable the membrane potential), or they open/close in response to a stimuli (e.g. the depolarization of a a neuron, that causes an electric effect, which in turn is sensed by the channel). So this does not happen when there is a large concentration of sodium/chloride ions outside the cell.
2. water will start to go out from the cell
Yes.
3. some ion may enter the cell which will increases the concentration of ion (Na+) and (Cl-). that will force the cell to close its ion channels because the transport process has one goal is to keep the condiction inside the cell very good for cell function.
No, the cellular membrane prevents the diffusion of ions. And as there is no activation/opening of Na/Cl channels - there will be no flow of ions into the potato cells.
this point i am very confused with. where the cell have ion channels which is open in normal conditions. the cell is placed in the salt solution may get in ( due to diffusion) but then close immediately because the cell will want to have more than what it need of ions so no harm came from having to much ions
No these channels are not always open. It they were open all the time, there would not be a difference in the concentration of ions inside the cell vs. the outside. The channels do instead open only when needed, and the control of the opening/closing is strictly regulated - otherwise chaos would rule and the ion concentration would be equalized between the outside and inside of the cell.
And the main purpose of the cellular membrane is to make it possible to have a stable and defined chemical environment inside the cell - pretty much regardless of the outside condition (within reason of course, in the biological context this works, but not if you supply a very salty solution or solvents etcetera).
4. the cell will stop sending water out of the cell by closing the aquaporins but it the cell was not able to stop the water from slipping away then the cell will be damaged the cell.
The aquaporins cannot be closed, so water will keep escaping the cell by osmosis, till the water concentration has been equilibrated (or more precise till osmosis stops, as a consequence the amount of osmotically active particles is equal on the respective sides of the cellular membrane).
according to Illinois university (https://www.ks.uiuc.edu/Research/aquaporins/) Gating of Water Channels so what do u think.
Well kind of, at some specific conditions some aquaporins behave this way, from their page:
" Although many aquaporins function as always-open channels, a subgroup of aquaporins, particularly in plants have evolved a sophisticated molecular mechanism through which the channel can be closed in response to harsh conditions of the environment, under which exchange of water can be harmful for the organism. Examples of such conditions are drought stress and flooding, which trigger certain cellular signals (dephosphorylation and change of pH) that result in closure of the channel."
This process is not omnipresent in all types of plant cells, and is a slow adaptation process to a slow change in outside water concentration. This demands sensing of a changed condition, signaling, dehposhorylation etcetera, to close these channels, so not really fast enough to keep water inside the potato cell during osmosis.
mag1 skrev:Stephan70707 skrev:
mag1 skrev:Stephan70707 skrev:Thank you again.
I understand the difference between osmosis and diffusion.
maybe it is my fault for explain my question very clearly.
Here i go.
let say we put cell inside salt soultion (NaCl) which has higher concentration of ion (Na+) and (Cl-).
the step that i belive could happen are.
1. At first few seconds, the cell has all there ions channel open ( if not all almost) especially aquaporins
No, ion channels do not open in response to a higher concentration of ions outside the cell. You are probably mixing this up with the ion conductance channels we have in the nerve cells of our bodies. One type of these the voltage gated channels do respond to a change in voltage over the membrane, by opening. This opening is however resulting from an electric effect (as during effect e.g. a nerve impulse). A concentration difference of does not result in a electric effect, in particular as the respective charges on the outside are balanced.
Aquaporins on the other are always open, and do thereby allow water molecules to diffuse out of the cells into the surrounding solution with a higher concentration of salt - i.e. osmosis occurs.
In book IRIS biologi 2 (sidan 70) says:" Olika kanalproteiner är anpassade för transport av speciella ämnen och vanligen kan de öppnas eller stängas efter behov. Kanaler för joner kallas även jonkanaler.
Yes these channels are essential for establishing and maintaning the normal state of the cell (the so called cellular homoeostasis). These channels do indeed transport ions through the membrane, but in a strictly regulated way by tight regulation - as not to disrupt the homeostasis. But these channels, and the transport systems they are part of, are not capable of coping with this type of (salt induced) osmosis.
These channels are either opening/closing to sustain the homeostasis (e.g. leak channels in neurons, trying to keep a stable the membrane potential), or they open/close in response to a stimuli (e.g. the depolarization of a a neuron, that causes an electric effect, which in turn is sensed by the channel). So this does not happen when there is a large concentration of sodium/chloride ions outside the cell.
can diffusion of O2 and CO2 happend here where those can diffuse through cell membrane?
2. water will start to go out from the cell
Yes.
3. some ion may enter the cell which will increases the concentration of ion (Na+) and (Cl-). that will force the cell to close its ion channels because the transport process has one goal is to keep the condiction inside the cell very good for cell function.
No, the cellular membrane prevents the diffusion of ions. And as there is no activation/opening of Na/Cl channels - there will be no flow of ions into the potato cells.
this point i am very confused with. where the cell have ion channels which is open in normal conditions. the cell is placed in the salt solution may get in ( due to diffusion) but then close immediately because the cell will want to have more than what it need of ions so no harm came from having to much ions
No these channels are not always open. It they were open all the time, there would not be a difference in the concentration of ions inside the cell vs. the outside. The channels do instead open only when needed, and the control of the opening/closing is strictly regulated - otherwise chaos would rule and the ion concentration would be equalized between the outside and inside of the cell.
And the main purpose of the cellular membrane is to make it possible to have a stable and defined chemical environment inside the cell - pretty much regardless of the outside condition (within reason of course, in the biological context this works, but not if you supply a very salty solution or solvents etcetera).
4. the cell will stop sending water out of the cell by closing the aquaporins but it the cell was not able to stop the water from slipping away then the cell will be damaged the cell.
The aquaporins cannot be closed, so water will keep escaping the cell by osmosis, till the water concentration has been equilibrated (or more precise till osmosis stops, as a consequence the amount of osmotically active particles is equal on the respective sides of the cellular membrane).
according to Illinois university (https://www.ks.uiuc.edu/Research/aquaporins/) Gating of Water Channels so what do u think.
Well kind of, at some specific conditions some aquaporins behave this way, from their page:
" Although many aquaporins function as always-open channels, a subgroup of aquaporins, particularly in plants have evolved a sophisticated molecular mechanism through which the channel can be closed in response to harsh conditions of the environment, under which exchange of water can be harmful for the organism. Examples of such conditions are drought stress and flooding, which trigger certain cellular signals (dephosphorylation and change of pH) that result in closure of the channel."
This process is not omnipresent in all types of plant cells, and is a slow adaptation process to a slow change in outside water concentration. This demands sensing of a changed condition, signaling, dehposhorylation etcetera, to close these channels, so not really fast enough to keep water inside the potato cell during osmosis.
Yes those gases do freely diffuse through the cell membrane.
okey so my thought is right.
as far as i understand well ... if we put potato in tap water with salt NaCl
1. they will be osmosis through vatten kanaler but aslo through cell membran (which can be very slow compared to vattenkanale)
2. they are diffusion of O2 and CO2 and other
3. thre are no diffusion for ion Na+ and CL-
4. there can be diffusion for other particlas such as Ions (Na+ and CL-) not that are within tap water and the cells ( in very small amount)
Note: tap water include many particles and many ions.
what do u think ?
Stephan70707 skrev:okey so my thought is right.
as far as i understand well ... if we put potato in tap water with salt NaCl
1. they will be osmosis through vatten kanaler but aslo through cell membran (which can be very slow compared to vattenkanale)
Yes, but the contribution from diffusion through the membrane is quite modest.
2. they are diffusion of O2 and CO2 and other
Yes.
3. thre are no diffusion for ion Na+ and CL-
Right.
4. there can be diffusion for other particlas such as Ions (Na+ and CL-) not that are within tap water and the cells ( in very small amount)
Note: tap water include many particles and many ions.
Some ions/particles in tap water, however this is already a pretty pure solution (for biology at least).

